Chronic and non-healing skin and wound infections represent a major and growing burden on global healthcare systems. These infections are frequently polymicrobial, involving complex communities of bacteria that cooperate, compete, and adapt within the host environment. Standard antibiotics are often ineffective in these settings due to poor penetration, biofilm formation, toxin-mediated tissue damage, and the rapid emergence of antimicrobial resistance (AMR). This project addresses these challenges by developing nanomedicine-based and host-inspired antimicrobial strategies specifically designed for polymicrobial skin and wound infections. We aim to deliver therapies that are not only effective against resistant pathogens but also promote tissue protection, reduce inflammation, and support healing.
Our lab approach recognises that successful treatment of chronic infections requires more than bacterial killing alone. In polymicrobial wounds, pathogens exploit host tissues through coordinated virulence mechanisms while benefiting from protective niches that shield them from antibiotics and immune clearance. We therefore focus on therapies that target both bacterial survival and virulence, while preserving host tissue integrity and limiting the selection of resistance.
Host-inspired anti-virulence approaches for tissue protection
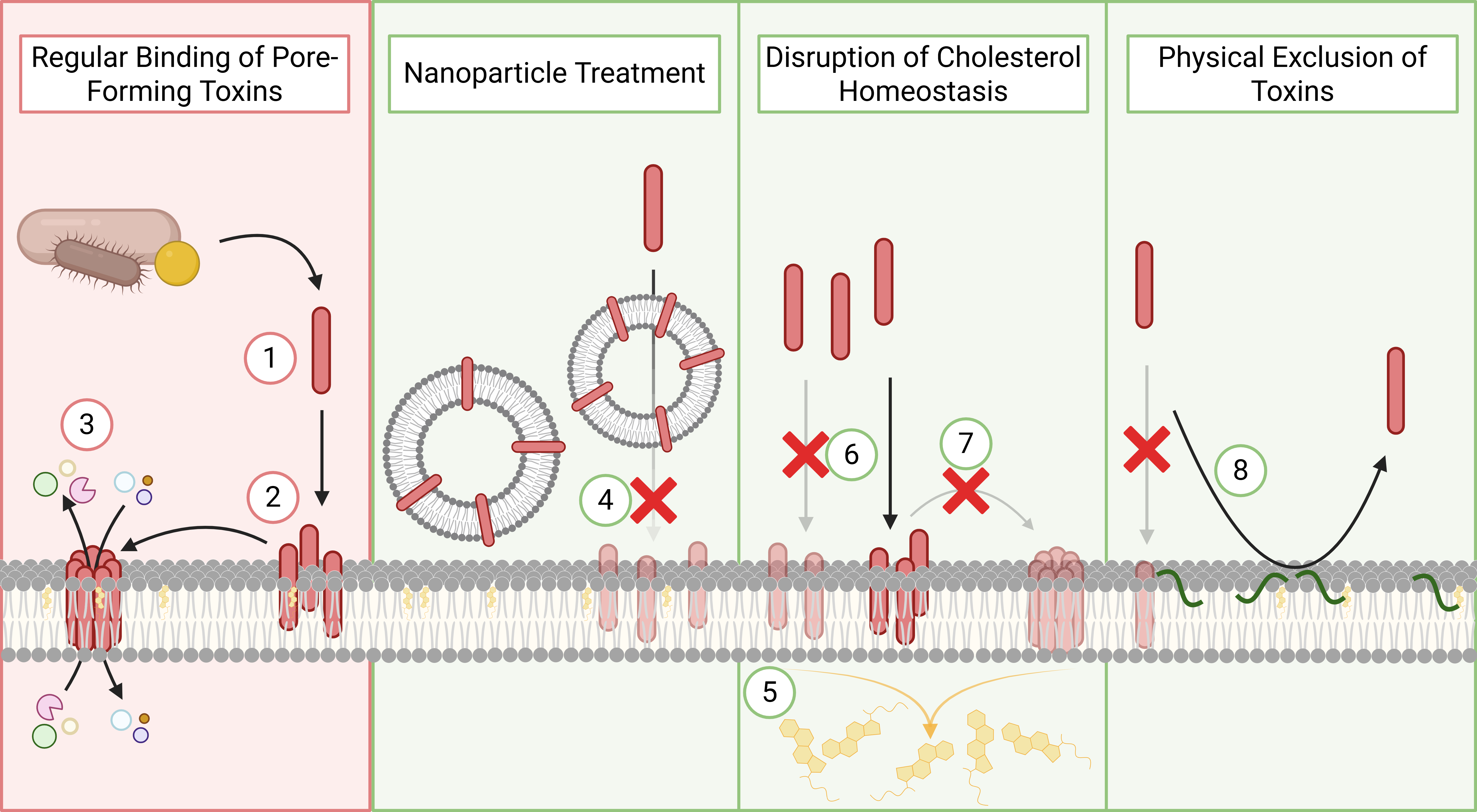
A central component of this project is the development of host-inspired anti-virulence strategies that protect skin and wound tissues from bacterial damage. Many clinically relevant wound pathogens, including Staphylococcus aureus and Pseudomonas aeruginosa, rely on membrane-targeting toxins and secreted virulence factors to disrupt host cells, exacerbate inflammation, and delay healing.
Rather than targeting these pathogens directly with high-dose antibiotics, we design therapies that neutralise bacterial toxins and reinforce host cell membranes, mimicking natural defence mechanisms. By reducing tissue damage and inflammation, these strategies improve the host’s ability to control infection and promote wound healing, while exerting minimal selective pressure for resistance. This makes them particularly well-suited for chronic and recurrent infections where resistance evolution is a persistent risk.
Smart nanomedicines for infection-specific drug activation
To overcome the limitations of systemic antibiotic delivery in complex infections, we develop stimuli-responsive nanomedicines and antimicrobial prodrugs that are activated selectively within infected tissues. Infections create unique microenvironments characterised by bacterial enzymes, altered redox conditions, and metabolic by-products. We exploit these cues to trigger site-specific antibiotic activation, ensuring that potent antimicrobial activity is concentrated at the infection site.
This strategy enables effective treatment of deeply embedded bacteria and biofilms while reducing off-target exposure to healthy tissue and commensal microbiota. By limiting unnecessary antibiotic activity, these systems directly address one of the key drivers of AMR in chronic wound care.
Nanomaterial platforms for polymicrobial infection Control
The project further explores nanomaterial-based platforms that combine antimicrobial delivery with direct interactions at the bacterial–host interface. These materials are engineered to enhance drug penetration into biofilms, disrupt polymicrobial community structure, and synergise with host immune responses.
By tuning nanoparticle size, surface chemistry, and responsiveness, we generate adaptable platforms suitable for topical or local delivery in skin and wound settings. These systems are designed with translational potential in mind, supporting future development into advanced wound dressings, localised therapies, or combination treatments that integrate seamlessly into existing clinical workflows.
Read more about our recent research here:
- Smith OK, Wise LM, Simcock J, Wannigama DL, Babiychuk EB, Pletzer D. Host-inspired anti-virulence strategies against membrane-targeting bacterial toxins: modification and mimicry. Crit Rev Microbiol. 2025 Dec 25:1-19. doi: 10.1080/1040841X.2025.2605546.
- MP Kabiraz, A Ali, D Pletzer. Advanced antimicrobial peptide-based biomaterials for food safety applications. LWT. 2025. 118884
- Ross CL, Lawer A, Sircombe KJ, Pletzer D, Gamble AB, Hook S. Site-Specific Antimicrobial Activity of a Dual-Responsive Ciprofloxacin Prodrug. J Med Chem. 2024 Jun 13;67(11):9599-9612. doi: 10.1021/acs.jmedchem.4c00724.
- Pletzer D, Mansour SC, Hancock REW. 2018. Synergy between conventional antibiotics and antibiofilm peptides in a murine, sub-cutaneous abscess model caused by recalcitrant ESKAPE pathogens. PLoS Pathog. 14(6):e1007084